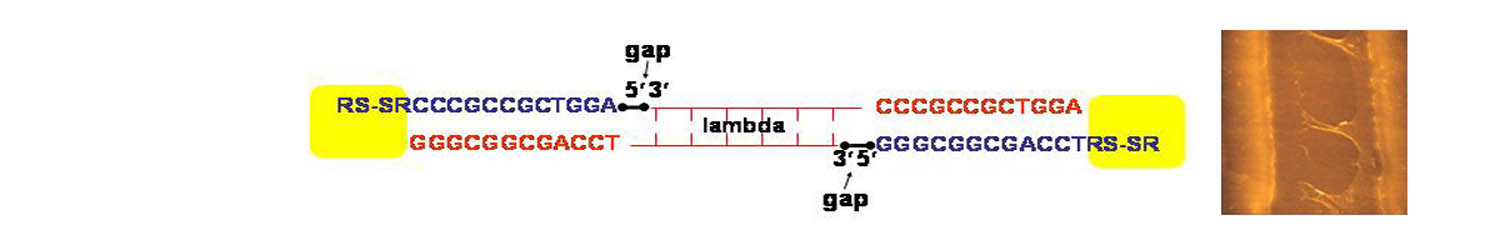
Biomolecular systems; DNA

To probe the relationship of structure to DNA electrical
properties, we have investigated linear lambda DNA molecules functionalized
with disulfide groups at both ends. The procedure of attaching disulfide
end groups to lambda DNA molecules generates two gaps or nicks in the
phosphate backbone, between the DNA and the short oligonucleotide segment
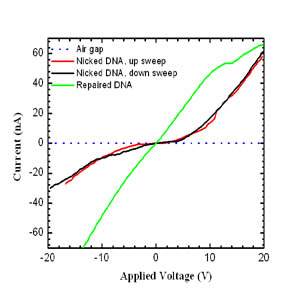
(indicated on the left above). These nicks can be repaired by the addition
of a ligation enzyme, and the I-V characteristics shown on the left reveal
a change in curve shapes as a function of structure. The repaired DNA
double helices show a close-to-linear I-V characteristic, with a DC
conductivity estimated at ~ 1x10-3 S cm-1. In contrast, the nicked lambda
DNA shows pronouncedly non-linear and rectifying behavior, with a
conductivity gap of ~ 3 eV. The low-field conductivity of the nicked
lambda DNA is approximately a factor 10 lower than the repaired lambda
DNA’s conductivity. [B. Hartzell et al. “Comparative current-voltage
characteristics of nicked and repaired lambda-DNA”, Applied Physics
Letters 82, 4800 (2003)].
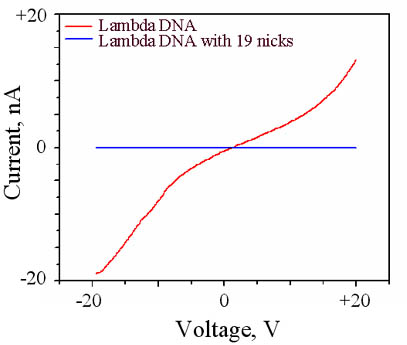
We have further investigated the effect of nicks in the
lambda backbone on charge transport. Nineteen nicks were introduced
using the artificial endonuclease, N.Bpu101, which recognizes a double
stranded DNA sequence but instead of cutting both strands it nicks the
phosphate backbone of only one of the strands at the nineteen recognition
sites present in lambda DNA. Comparative I-V measurements of lambda
DNA versus lambda DNA with 19 nicks is shown in the figure on the left.
The introduction of 19 nicks reduces the magnitude of the measured
current to that of the airgap, again indicating to the interplay between
structure and electronic properties of DNA.
 To assess the influence of disulfide termination on charge transport,
we have synthesized lambda DNA molecules labeled with disulfide end groups in two
configurations: at the 3’ ends of opposing strands, or on the 3’ and 5’ ends of the
same strand. In the latter configuration, only one strand is attached and contacted
to the two Au electrodes utilized in the measurement. I-V measurements of these two
types of lambda DNA show no appreciable differences in curve shapes, indicating that
the position of the disulfide is less important than the fidelity of the double helical
structure. [B. Hartzell et al. “Current-voltage characteristics of diversely disulfide
terminated labmda-DNA” Journal of Applied Physics 95, 2764 (2003)].
To assess the influence of disulfide termination on charge transport,
we have synthesized lambda DNA molecules labeled with disulfide end groups in two
configurations: at the 3’ ends of opposing strands, or on the 3’ and 5’ ends of the
same strand. In the latter configuration, only one strand is attached and contacted
to the two Au electrodes utilized in the measurement. I-V measurements of these two
types of lambda DNA show no appreciable differences in curve shapes, indicating that
the position of the disulfide is less important than the fidelity of the double helical
structure. [B. Hartzell et al. “Current-voltage characteristics of diversely disulfide
terminated labmda-DNA” Journal of Applied Physics 95, 2764 (2003)].
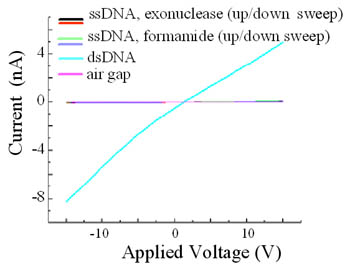
To further evaluate the influence of the double helical structure, we measure the I-V
characteristics of double stranded (dsDNA) versus single-stranded (ssDNA) molecules.
For these measurements, we utilize lambda DNA molecules that possess disulfide linkers
on the 3’ and 5’ ends of the same strand. This allows us to first measure lambda in
the double stranded form, and then single strand the molecules on the device itself
and compare the results. The ssDNA was formed from the dsDNA using two different
methods: a thermal/chemical denaturation and enzymatic digestion utilizing lambda
exonuclease. Resulting I-V characteristics for both the double stranded and single
stranded samples are close-to-linear when measured at room temperature. However,
the ssDNA samples consistently give conductivity values about a factor 50 smaller
in amplitude, as seen in the figure on the left. These observations reinforce the
importance of the double helical structure in DNA charge transport. Support from NSF
NIRT grant 0103034.