Research Projects
Zeolite-like materials for energy applications
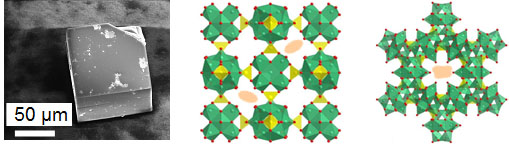 Electrically conducting zeolite-like frameworks are largely unstudied as electronic materials,
but may offer new avenues in energy applications, ranging from electrical energy storage to catalysis.
Zeolite-like materials are characterized by the presence of nanoscale channels and pores delineated by their
crystalline framework. Here, we present a 3-dimensional oxo-vanadium arsenate with framework composition [(As6V15O51)-9],
that exhibits mixed conductivity -ionic and electronic. The figure on the right denotes an SEM image of a single crystals of the material (left),
the structure down the a axis with the template shown as ellipses (middle), and the view down the 111 direction (right).
The electrical measurements are performed on single crystals.
Electrically conducting zeolite-like frameworks are largely unstudied as electronic materials,
but may offer new avenues in energy applications, ranging from electrical energy storage to catalysis.
Zeolite-like materials are characterized by the presence of nanoscale channels and pores delineated by their
crystalline framework. Here, we present a 3-dimensional oxo-vanadium arsenate with framework composition [(As6V15O51)-9],
that exhibits mixed conductivity -ionic and electronic. The figure on the right denotes an SEM image of a single crystals of the material (left),
the structure down the a axis with the template shown as ellipses (middle), and the view down the 111 direction (right).
The electrical measurements are performed on single crystals.
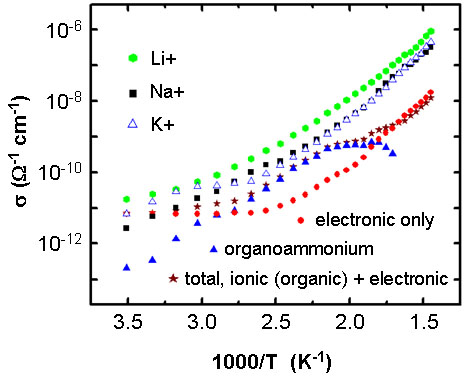 The material is zeolite-like and the framework is stable towards intercalation reactions;
the organic template and water molecules can be reversibly exchanged with organic or inorganic cations.
Electrical measurements are performed after contacting individual crystals down to ~ 50 Ám on a side by silver-epoxy.
The room temperature conductivity measured for a cube with 50 micrometer edge is 3 x10-8 Ohm-1 cm-1 .
We investigate the contributions and interactions of the parallel ionic and electronic conduction pathways through
measurement of the electrical conductivity as a function of temperature (T).
We measure the total conductivity due to the framework plus the extra-framework constituents, and the electronic conductivity
due to the empty framework, and obtain the ionic conductivity by subtraction.
The figure on the left shows the variable temperature conductivity of the material in its as synthesized form and in its ion-exchanged form.
The comparative plot highlights the electronic (circle) contribution to the total conductivity (star).
The temperature range is 285 to 690 K.
The material is zeolite-like and the framework is stable towards intercalation reactions;
the organic template and water molecules can be reversibly exchanged with organic or inorganic cations.
Electrical measurements are performed after contacting individual crystals down to ~ 50 Ám on a side by silver-epoxy.
The room temperature conductivity measured for a cube with 50 micrometer edge is 3 x10-8 Ohm-1 cm-1 .
We investigate the contributions and interactions of the parallel ionic and electronic conduction pathways through
measurement of the electrical conductivity as a function of temperature (T).
We measure the total conductivity due to the framework plus the extra-framework constituents, and the electronic conductivity
due to the empty framework, and obtain the ionic conductivity by subtraction.
The figure on the left shows the variable temperature conductivity of the material in its as synthesized form and in its ion-exchanged form.
The comparative plot highlights the electronic (circle) contribution to the total conductivity (star).
The temperature range is 285 to 690 K.
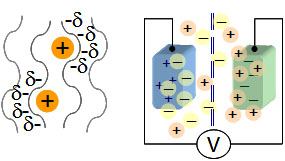 In an electrically conducting zeolite-like material, an electrical double-layer may form
between a charge in a pore and the framework, resulting in electrical double-layer capacitive electrical
energy storage.
The ionic electrical double-layer (EDLC) forms the basis for ultracapacitors, here formed between mobile
ions in an electrolyte and electrons in a conducting substrate.
The capacitance per unit area is high, due to the atomic charge separation distance typical
of EDLCs (widespread electrolytic capacitors). Ultracapacitor adds to this a large effective area of the
capacitor plate by employing a porous conducting medium as electrode.
Again, left panel in figure illustrates how the double-layer concept carries over to a zeolite-like material,
where a positive charge residing in a zeolite pore may induce a distribution of compensating charges from electrons
in the framework.
The schematic concept of an EDLC with two conducting zeolite-like frameworks attached to an external voltage source,
is depicted in the right panel (figure on the right).
An ion membrane is located in-between, permeable to the electrolyte ions. One framework is positively charged and
hosts negative ions inside its structure, the other vice-versa.
Experimentally, we set up a model consisting of two single crystals immersed in KCL solution. Modeling the set-up with circuit
elements to best fit to the observed curves, we deduce that the framework shows an expected improvement in
capacitance over mesoporous carbon.
In an electrically conducting zeolite-like material, an electrical double-layer may form
between a charge in a pore and the framework, resulting in electrical double-layer capacitive electrical
energy storage.
The ionic electrical double-layer (EDLC) forms the basis for ultracapacitors, here formed between mobile
ions in an electrolyte and electrons in a conducting substrate.
The capacitance per unit area is high, due to the atomic charge separation distance typical
of EDLCs (widespread electrolytic capacitors). Ultracapacitor adds to this a large effective area of the
capacitor plate by employing a porous conducting medium as electrode.
Again, left panel in figure illustrates how the double-layer concept carries over to a zeolite-like material,
where a positive charge residing in a zeolite pore may induce a distribution of compensating charges from electrons
in the framework.
The schematic concept of an EDLC with two conducting zeolite-like frameworks attached to an external voltage source,
is depicted in the right panel (figure on the right).
An ion membrane is located in-between, permeable to the electrolyte ions. One framework is positively charged and
hosts negative ions inside its structure, the other vice-versa.
Experimentally, we set up a model consisting of two single crystals immersed in KCL solution. Modeling the set-up with circuit
elements to best fit to the observed curves, we deduce that the framework shows an expected improvement in
capacitance over mesoporous carbon.
 Electrically conducting zeolite-like frameworks are largely unstudied as electronic materials,
but may offer new avenues in energy applications, ranging from electrical energy storage to catalysis.
Zeolite-like materials are characterized by the presence of nanoscale channels and pores delineated by their
crystalline framework. Here, we present a 3-dimensional oxo-vanadium arsenate with framework composition [(As6V15O51)-9],
that exhibits mixed conductivity -ionic and electronic. The figure on the right denotes an SEM image of a single crystals of the material (left),
the structure down the a axis with the template shown as ellipses (middle), and the view down the 111 direction (right).
The electrical measurements are performed on single crystals.
Electrically conducting zeolite-like frameworks are largely unstudied as electronic materials,
but may offer new avenues in energy applications, ranging from electrical energy storage to catalysis.
Zeolite-like materials are characterized by the presence of nanoscale channels and pores delineated by their
crystalline framework. Here, we present a 3-dimensional oxo-vanadium arsenate with framework composition [(As6V15O51)-9],
that exhibits mixed conductivity -ionic and electronic. The figure on the right denotes an SEM image of a single crystals of the material (left),
the structure down the a axis with the template shown as ellipses (middle), and the view down the 111 direction (right).
The electrical measurements are performed on single crystals.
 The material is zeolite-like and the framework is stable towards intercalation reactions;
the organic template and water molecules can be reversibly exchanged with organic or inorganic cations.
Electrical measurements are performed after contacting individual crystals down to ~ 50 Ám on a side by silver-epoxy.
The room temperature conductivity measured for a cube with 50 micrometer edge is 3 x10-8 Ohm-1 cm-1 .
We investigate the contributions and interactions of the parallel ionic and electronic conduction pathways through
measurement of the electrical conductivity as a function of temperature (T).
We measure the total conductivity due to the framework plus the extra-framework constituents, and the electronic conductivity
due to the empty framework, and obtain the ionic conductivity by subtraction.
The figure on the left shows the variable temperature conductivity of the material in its as synthesized form and in its ion-exchanged form.
The comparative plot highlights the electronic (circle) contribution to the total conductivity (star).
The temperature range is 285 to 690 K.
The material is zeolite-like and the framework is stable towards intercalation reactions;
the organic template and water molecules can be reversibly exchanged with organic or inorganic cations.
Electrical measurements are performed after contacting individual crystals down to ~ 50 Ám on a side by silver-epoxy.
The room temperature conductivity measured for a cube with 50 micrometer edge is 3 x10-8 Ohm-1 cm-1 .
We investigate the contributions and interactions of the parallel ionic and electronic conduction pathways through
measurement of the electrical conductivity as a function of temperature (T).
We measure the total conductivity due to the framework plus the extra-framework constituents, and the electronic conductivity
due to the empty framework, and obtain the ionic conductivity by subtraction.
The figure on the left shows the variable temperature conductivity of the material in its as synthesized form and in its ion-exchanged form.
The comparative plot highlights the electronic (circle) contribution to the total conductivity (star).
The temperature range is 285 to 690 K.
 In an electrically conducting zeolite-like material, an electrical double-layer may form
between a charge in a pore and the framework, resulting in electrical double-layer capacitive electrical
energy storage.
The ionic electrical double-layer (EDLC) forms the basis for ultracapacitors, here formed between mobile
ions in an electrolyte and electrons in a conducting substrate.
The capacitance per unit area is high, due to the atomic charge separation distance typical
of EDLCs (widespread electrolytic capacitors). Ultracapacitor adds to this a large effective area of the
capacitor plate by employing a porous conducting medium as electrode.
Again, left panel in figure illustrates how the double-layer concept carries over to a zeolite-like material,
where a positive charge residing in a zeolite pore may induce a distribution of compensating charges from electrons
in the framework.
The schematic concept of an EDLC with two conducting zeolite-like frameworks attached to an external voltage source,
is depicted in the right panel (figure on the right).
An ion membrane is located in-between, permeable to the electrolyte ions. One framework is positively charged and
hosts negative ions inside its structure, the other vice-versa.
Experimentally, we set up a model consisting of two single crystals immersed in KCL solution. Modeling the set-up with circuit
elements to best fit to the observed curves, we deduce that the framework shows an expected improvement in
capacitance over mesoporous carbon.
In an electrically conducting zeolite-like material, an electrical double-layer may form
between a charge in a pore and the framework, resulting in electrical double-layer capacitive electrical
energy storage.
The ionic electrical double-layer (EDLC) forms the basis for ultracapacitors, here formed between mobile
ions in an electrolyte and electrons in a conducting substrate.
The capacitance per unit area is high, due to the atomic charge separation distance typical
of EDLCs (widespread electrolytic capacitors). Ultracapacitor adds to this a large effective area of the
capacitor plate by employing a porous conducting medium as electrode.
Again, left panel in figure illustrates how the double-layer concept carries over to a zeolite-like material,
where a positive charge residing in a zeolite pore may induce a distribution of compensating charges from electrons
in the framework.
The schematic concept of an EDLC with two conducting zeolite-like frameworks attached to an external voltage source,
is depicted in the right panel (figure on the right).
An ion membrane is located in-between, permeable to the electrolyte ions. One framework is positively charged and
hosts negative ions inside its structure, the other vice-versa.
Experimentally, we set up a model consisting of two single crystals immersed in KCL solution. Modeling the set-up with circuit
elements to best fit to the observed curves, we deduce that the framework shows an expected improvement in
capacitance over mesoporous carbon.