Properties of Bubbles
This page serves as a location where the information pertaining to the surface tension of films and liquids is stored. This includes films at a liquid interface, liquids at a solid interface, and a film at a solid interface.
This page serves as a location where the information pertaining to the surface tension of films and liquids is stored. This includes films at a liquid interface, liquids at a solid interface, and a film at a solid interface.
Pure liquids cannot sustain bubbles on their surface. This is due to the fact that they have too little surface tension. For bubbles to form, surfactants must be present in the bubble forming solution. In the case of a soap bubble the surfactant is, of course, soap. Soap and many other surfactants generally consist of a metal salt with a long fatty acid tail attached to it. When in water, the salt ionizes, leaving the tail attached to one of the ions, consistently of the same charge. These ions feel a net force towards the surface as the fatty acids are hydrophobic. The chemical interaction of the ions at the surface create a higher surface tension than there otherwise would be. This increased surface tension allows the solution to hold its shape even after most of the interior solution has drained out of the bubble.
Surface tension exists because at the surface of the liquid, solid, or gas there is a difference in pressure from the rest of the material. To begin the definition, assume a Lennard-Jones potential for the interaction between atoms. In the volume of the material, there is more pressure from the surroundings, pressing the molecules closer to each other, so that the force they put on each other is slightly repulsive. However, on the surface of the material, there is much less pressure, and so the atoms are farther apart, creating a more attractive interaction. This stronger attraction is what gives rise to surface tension.
Since surface tension is a force that lies tangent to the surface, a difference in surface tension across the surface results in the surface moving. This is most easily seen in the changing colors of a soap bubble. The inconsistencies on the surface move due to the surface tension, causing a change in the local thickness of the bubble and thus the color yielded by the thin-film light interference. As discussed in later sections, the surface tension of a bubble, in conjunction with the pressure difference inside and outside of the bubble, are important factors in determining its shape.
In thin films, surface tension plays a very important role in the shape that the film takes. The Young-Laplace Equation, which relates the relative pressures the two sides of a film to its curvature, is proportional to the surface tension of the film.
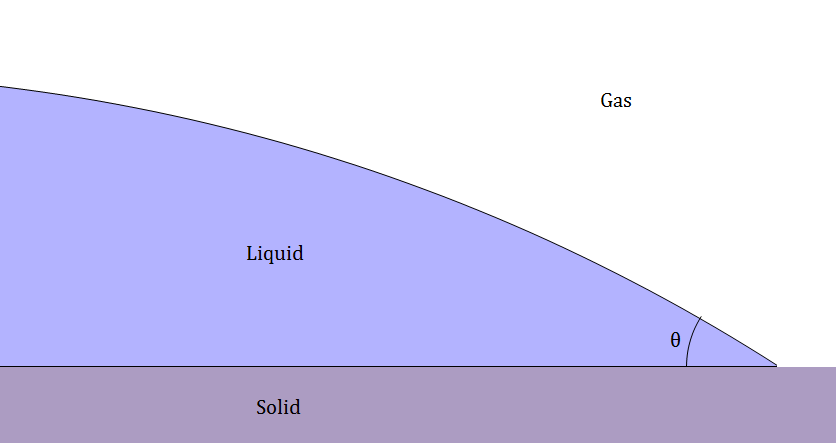
The contact angles between a solid and a liquid that are surrounded by gas (such as a drop of water on a plate) can be described by $$\gamma_{s-g} = \gamma_{s-l} + \gamma_{g-l} \cos(\theta)$$ where γs-g is the surface tension between the solid and the gas, γs-l is the surface tension between the solid and the liquid, and γg-l is the surface tension between the gas and the liquid. This equation does not serve very well to predict the contact angles between a solid and a liquid as it is generally more difficult than it is worth to determine the surface tension of the materials in question.
Because the contact angles in question are films contacting other films, this case is actually simpler than with a solid surface, due to the similarity of the contacting materials. These films contact symmetrically with one another at 120 degrees. This is further discussed in the section Plateau Laws.
Film solid Contact Angles are at a junction where the thin film meets a solid.
While undeformed in the volume of a liquid, bubbles try to maintain a spherical shape. The driving force behind this shape is the surface tension of the bubble interface. This is attempting to minimize the surface area of the bubble. For an enclosed surface with no boundary conditions, only a fixed volume, the shape with the minimum surface area is a sphere.
On a dry surface, meaning there is little liquid between the interfaces in the film, the bubble has 2 components. The top is a spherical cap with a radius dictated by the Young Laplace Equation, . The bottom of this bubble, which is submerged in the solution, is a deformed spherical cap. As the depth at which the bubble rests increases, the buoyant force increases. This causes the bubble to flatten out. This deformation is currently a topic of research in Dr. Cheng's and Dr. Feitosa's groups, and its exact shape as a function of the material parameters of the bubble. In a dry bubble, the interface between these two portions of the bubble can be considered disjoint, although in reality there is a continuous change between the two.
1Stefan Hutzler and Denis Weaire The Physics of Foams, (Oxford University Press, New York, 1999).