RESEARCH INTERESTS
Structure and dynamics of aqueous solvation shells
Water plays an important role in chemical and biological processes. In aqueous solutions, water molecules dissolve ionic compounds, charged chemical species by forming hydration shells around them. Here we have employed a vector network analyzer-based spectrometer to measure the complex dielectric response of liquid water and aqueous solutions with unprecedented precision over the range 0.5 GHz to 1.1 THz. In doing so, we have obtained statistically significant evidence that the dynamics of water over this frequency regime are best described in terms of three Debye relaxation processes with the characteristic times of 8.56, 1.10 ps and 179 fs (at 25.0oC). The relaxation time and the amplitude of these processes allow us to understand the structure and the dynamics of water in the hydration shells around ions.
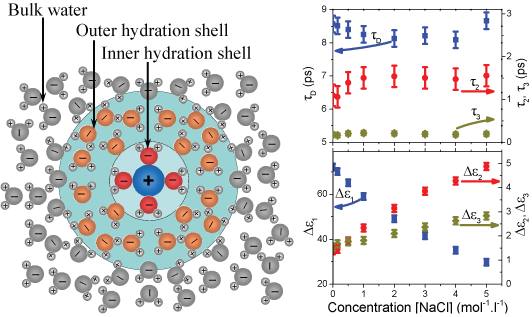
(left) Due to the high polarity, water molecules close to the dissolved ions
from the hydration shells. (right) The relaxation time constants and amplitudes
vary with salt concentration provide insights into the mechanisms underlying
these dynamic processes. The time constants of all three Debye processes are
effectively independent of salt concentration. Their amplitudes, in contrast,
vary significantly with changing salt concentration: while the amplitude of the
slowest relaxation falls with rising salt concentrations, the amplitudes of the
faster components increase with increasing salt concentration.
